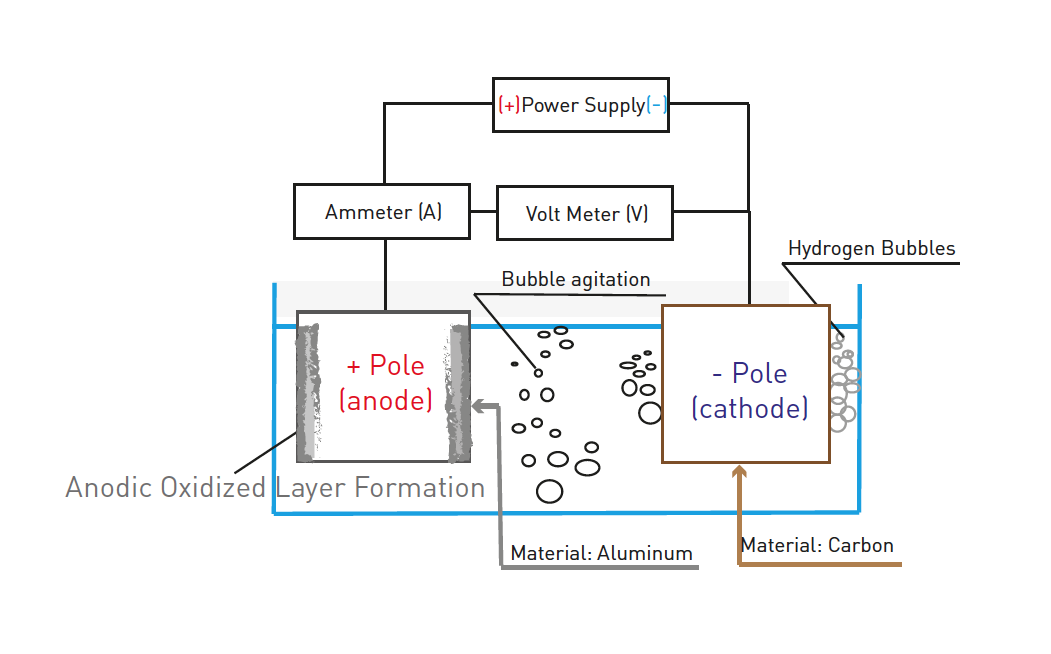
Anodizing is produced by electrochemical conversion. In an anodizing process, the metal workpiece to be anodized is the anode in a suitable electrolyte. With the electric current passing through the electrolyte, the metal surface is converted to an oxide form. An anodizing process is usually used on aluminum (magnesium, titanium and other nonferrous metals) for protection and cosmetic purposes.
The electrolyte provides oxygen ions that react with metal ions to form the oxide, and hydrogen is released at the metal or carbon cathode.
Anodizing differs from electroplating in two aspects: in electroplating, the workpiece to be plated is the cathode, and the metallic coatings are deposited on the workpiece. In anodizing, the workpiece is the anode, and its surface is converted to an oxide form.